GFI research grants update: Introduction to cell-based meat research projects

In February, we announced the winners of GFI’s inaugural Competitive Grant Program for critical plant-based and cell-based meat research. The fourteen selected research projects are now underway!
Part 1 of our update introduced the plant-based meat research projects. Now, read on to learn more about the cell-based meat research we funded.
The basics of cell-based meat production
Animal meat is muscle tissue that is composed of muscle cells, fat cells, and connective tissue cells (fibroblasts). By starting with a sample of cells from an animal, we can produce thousands of kilograms of meat without the need to slaughter animals. This cell-based meat is genuine animal meat—just produced much more efficiently.
The small starting sample of cells is fed the nutrients and water they need to grow and replicate. This food is called cell culture media. By modifying the environment that the cells are in, we can induce them to then differentiate into the muscle, fat, and connective tissue that make up meat. In order to provide the cells with instructions for how to organize themselves into the proper 3D structure, a support system (or scaffold) is added. All of this takes place in a cultivator (also called a bioreactor).
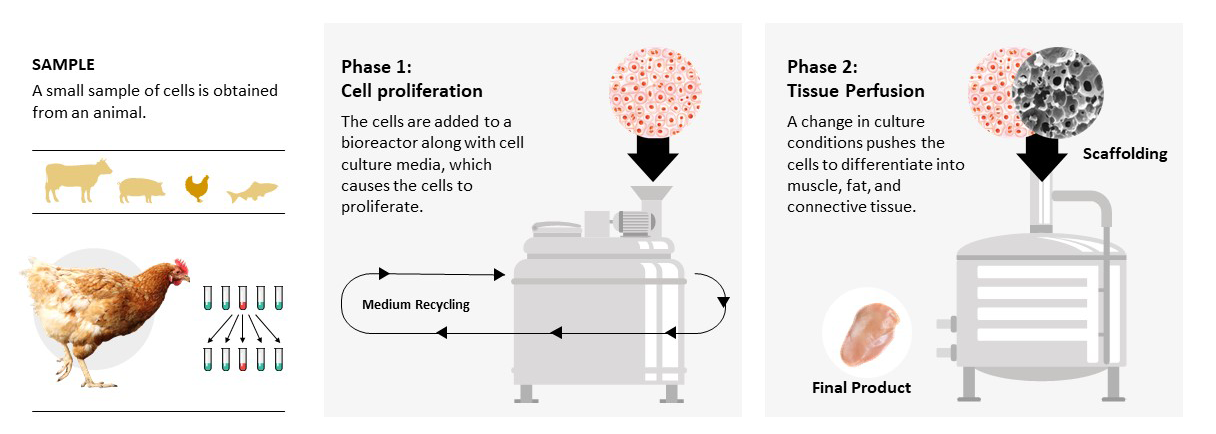
The cell-based meat industry has benefited from scientific knowledge and technical developments made in other industries that use cell lines, cell culture media, scaffolds, and cultivators. However, two key aspects of the cell-based meat industry—cost and scale—create novel technical challenges that must be addressed. The six projects we funded focus on critical technical barriers with an eye toward large-scale, low-cost cell-based meat production.
We’ll now briefly present the overall goals of each project and how they will enable more economic and efficient production of cell-based meat at commercial scale.
Cell line development
Very few academic labs are involved with cell-based meat research. One potential barrier to conducting research in this area is the need to first acquire cell lines for animals that are commonly consumed as food, including mammalian livestock and poultry. Suitable cell lines for many of these animal species are not available. Thus, most academic cell-based meat research must begin by dedicating time, effort, and resources to cell line creation. As more and more research labs begin to work in this field, it will be extremely inefficient for each lab to generate their own cell lines. By ensuring that a cell line repository is generated through standardized procedures and the cell lines are well-characterized, subsequent R&D utilizing these cell lines will experience less lab-to-lab and experiment-to-experiment variability.
Enter: The Frozen Farmyard. Gareth Sullivan (University of Oslo, Norway) is generating a comprehensive library of fibroblast cells (i.e., connective tissue cells) that will provide the basis of the Frozen Farmyard. Ultimately, these fibroblasts will serve as the source cells to produce induced pluripotent stem cell (iPSC) lines. iPSCs are cells from adult tissue that are reprogrammed back to a state where they can divide many times. They also have the potential to differentiate into all the mature cell types in the body, including muscle, fat, blood, and connective tissue (all the cell types that are important for cell-based meat).
Dr. Sullivan’s Frozen Farmyard will be an ever-expanding repository of cell lines. Having available cell lines from different species and a variety of breeds within species will enable insightful basic research for the cell-based meat industry. Creating these critical starting materials will open the door for other researchers to conduct cell-based meat research.
Cell culture media
Cell culture media contain many components including vitamins, minerals, amino acids, carbohydrates, lipids, and growth factors. (Growth factors are signaling proteins that stimulate cell division, growth, differentiation, survival, and other essential cellular functions.) The cells require a continual supply of these nutrients in order to proliferate and differentiate. However, cell culture media is currently very expensive and is expected to be one of the primary cost drivers for large-scale cell-based meat production. This is primarily due to the high cost of growth factors.
Peter Stogios (University of Toronto, Canada) is tackling this issue. By screening growth factor proteins from dozens of species and careful analysis of their structure and function, Dr. Stogios and his team will create novel animal-free growth factors that work better and cost less than existing growth factors. These new growth factors will be attractive tools for accelerating the scale-up of cell-based meat production.
Scaffolding and structuring
Meat comes in a variety of shapes and sizes, from ground beef products to chicken breasts and pork chops. One remaining challenge facing the cell-based meat industry is how to organize the mix of differentiated cells into the familiar, sinewy structures of conventional animal meat. Several cell-based meat companies have already succeeded in producing and taste-testing different ground meat products. The goal now is to create scalable methods for producing thicker cuts of meat like steaks.
Both Marcelle Machluf (Technion – Israel Institute of Technology, Israel) and Amy Rowat (UCLA, USA) are developing scaffolding technologies that will help produce larger, 3D cell-based meat structures. Prof. Machluf and her collaborators are creating small, edible scaffolds called microcarriers. These microcarriers will organize the cells into “building blocks” that can then be further arranged to create thicker cuts of meat. The technologies under investigation are cost-effective and easily scalable. Interestingly, the process can be utilized to create a variety of products ranging from minced cell-based meat to 3D cuts like cell-based chicken breasts. This means cell-based meat companies would be able to use the same “building blocks” to create a range of end products.
Dr. Rowat’s team is creating fiber-like scaffolds with regions of varying degrees of stiffness. The scaffolds will help arrange the cells into meaty fibers, and the variation in flexibility and stiffness will encourage muscle and fat cells to co-exist. Interspersing fat cells and muscle cells is critical for achieving a high-fidelity cell-based steak. Dr. Rowat’s research is a terrific example of the synergies that can arise from blending the plant-based and cell-based worlds. Her choice of starting materials for the scaffolds come from algae and fruit, have proven biocompatibility, and are environmentally friendly!
Cultivators and bioprocess
Cultivators are the devices that house the cells, cell culture media, and any required scaffolding structures. (At commercial scale, they will likely look like the beer brewing tanks you see at breweries.) Cultivators support the “bioprocess” of cell-based meat development, which is essentially the activity required for animal cells to be grown and harvested to produce meat.
Ivana Gadjanski (BioSense Institute, University of Novi Sad, Serbia) and her colleagues are creating low-cost sensors that will help cell-based meat manufacturers monitor the environment inside the cultivator. The sensors will provide real-time data on the growth of cells and the composition of the cell culture medium in the cultivator. This information will enable recycling of cell culture media and its components. For example, sensors can help determine when metabolic waste products (like ammonia) need to be filtered out. They can also identify when nutrients (like sugars) have been used up and need to be replenished. The sensing technologies developed by Dr. Gadjanski’s team will directly lead to increased efficiency and lower costs at production scale.
As mentioned earlier, cost and scale are two characteristics of cell-based meat production that are vastly different from today’s existing biomedical industry. Mariana Petronela (Petra) Hanga (Aston University, UK) is focused on addressing these bottlenecks in the commercialization of cell-based meat. Dr. Hanga’s research will develop bioprocesses that enable the reliable scale-up of muscle and fat cells in culture together. The protocols created will balance optimized cell growth with maintenance of the quality of both the muscle and fat cells. A cost of goods analysis will also allow the team to make recommendations about how to minimize cell-based meat production costs.
Together, these six projects address critical technical barriers facing the cell-based meat industry. These researchers are committed to sharing their research and results with all of us, and we cannot wait to see how their work will accelerate even more game-changing research and development aimed at bringing affordable, high-quality cell-based meat to consumers around the world.
Over the next few months, we’ll be giving GFI blog readers an inside look at each of these projects and the researchers behind them. If you’d like an opportunity to meet all of our 2018 grant awardees in person, register to join us at the 2019 Good Food Conference in September!

