Cultivated meat research just got a major boost. Here’s why it matters.

“If you want to practice biology, do it on the leading edge, and if you want to be on the leading edge, invent new tools for deciphering biological information.”
Those were the words given by William Dreyer to his graduate student Leroy Hood, who would go on to invent the technology behind DNA sequencing. The quote highlights the importance of tools in enabling researchers to answer questions and address challenges in scientific research.
And it’s why we’re excited about the potential of GFI’s acquisition of SCiFi Foods’ cell lines and growth media—critical research tools now available to the field—to unlock new discoveries and accelerate cultivated meat research.
Closing the gap
For cultivated meat, downstream success depends on having high-quality cell lines and media to enable efficient cell growth. Without this foundation, the field will struggle to develop cost-effective media formulations, scaffolds, bioreactors, and bioprocesses. And without these important assets, the generation of enough suitable tissue or biomass to inform product development and achieve desirable end-product taste, texture, and sensory profiles will remain out of reach.
Each of these developmental steps can take years and several million dollars to make meaningful progress. While some labs have successfully progressed in the first step—developing scalable cell lines that grow in serum-free media—the majority are still starting from scratch.
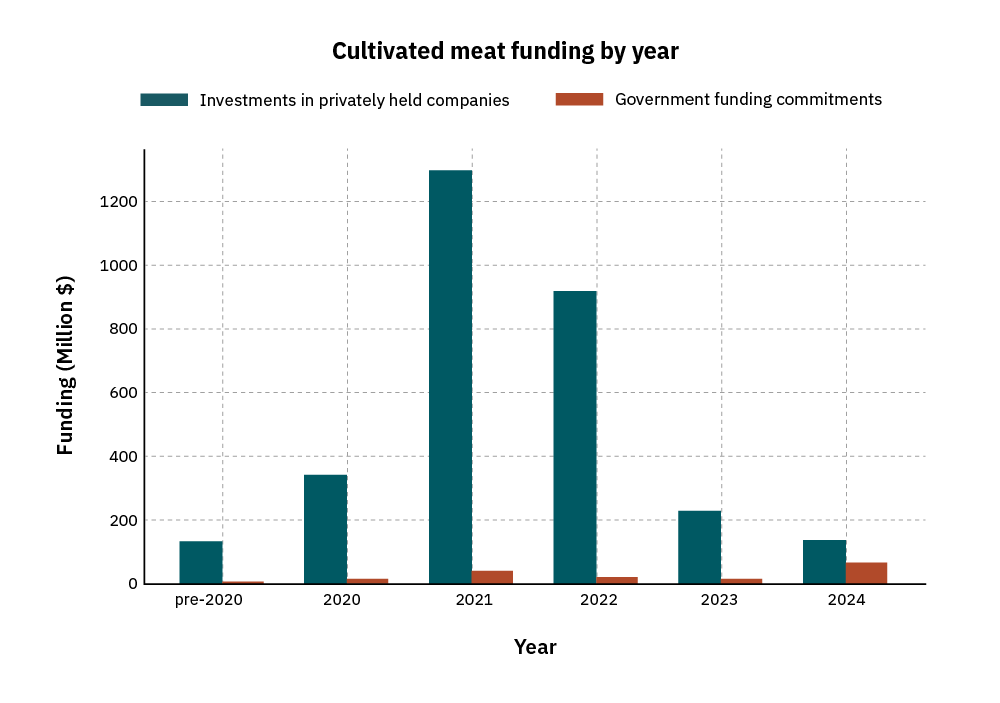
Since the cultivated meat sector began just over a decade ago, investments in privately held companies have significantly outpaced public investments in R&D, with a gap of ~$2.9B [Figure]. As a consequence, the field suffers in two ways: most academic research is still years away from being commercially relevant or applicable, and the public knowledge generated by academia lags several years behind the leading edge of industry knowledge. This gap makes it challenging to align academic and industry research priorities, and creates a misleading public information landscape that does not accurately represent cultivated meat’s technological readiness and potential.
The concept of “composting”
At GFI, we’re working to close this gap by enabling greater ecosystem-wide collaboration. If companies shared data, tools, failures, and successes, and worked more closely with academia and research centers through partnerships, the scientific field could advance in tandem and at a much quicker pace. But this is easier said than done.
Typically, cell lines, media formulations, and other process technologies remain proprietary. It’s understandable, given their foundational importance in providing a competitive advantage in the market.
So when we learned about cultivated meat startup SCiFi Foods’ auction of its assets, we knew we had a unique opportunity to lean into our role as a field catalyst and circumvent this roadblock. After GFI’s bid for multiple bovine cell lines and the required serum-free media was accepted, we partnered with the Tufts University Cellular Agriculture Commercialization Lab to bank and distribute the cells. Since our October 16 announcement, we’ve already seen interest from ten academic institutions and six companies, demonstrating the high value of these cell lines.
By composting these resources from startups winding down, we believe that academic labs, new startups, and existing B2B companies can leapfrog years of R&D. They are enabled to begin at a commercially relevant starting point for downstream development of scaffolds, processes, and end products using a shared, standard set of tools.
Why we’re excited

“I’m excited because these are the first suspension-adapted bovine cell lines that will be available to the research community. Some of these cell lines have already been grown at 500L scale, which means researchers can jump right into optimizing scale-up and process development, an area where we’ve only begun to scratch the surface.
I’m also excited about open-sourcing commercially relevant media formulations. The FSF4 media formulation, in particular, is serum-free, chemically defined, free of expensive proteins such as albumin, transferrin, and insulin, and was previously submitted to the FDA for safety evaluation. These milestones represent millions of dollars and years of R&D that can now be applied by anyone. Most media formulations are held as trade secrets, and those that are publicly available were often formulated for low-density, adherent cell culture.
This means they do not contain the correct amount of nutrition (i.e., amino acids, sugars) suitable for cultivated meat. Academic researchers often unwittingly assume public formulations such as DMEM/F12 will be used as-is in commercial production, leading to conclusions in their studies misaligned with commercial reality. Accordingly, the media formulation itself can inform more accurate cost modeling, environmental impact studies, and supply chain optimization.”
-ELLIOT SWARTZ, PHD, SENIOR PRINCIPAL SCIENTIST, CULTIVATED MEAT, GFI
“I’m excited because the composting effort is fundamentally aimed at increasing connectivity and minimizing redundancy in the alternative protein ecosystem. Opening up SciFi Foods’ cell line and media assets to the world encourages a common ground for R&D and enables a new level of comparison and transparency in results—an experiment performed in one country or lab can now be easily validated and built upon in another.
Such a democratization of commercially relevant tools is essential for speeding up process development; it allows the global community to stop endlessly reinventing the wheel in siloes and start building a cohesive and collaborative platform for innovation instead.
In the biopharmaceutical industry, “workhorse” cell lines, such as CHO, HEK, and BHK, emerged decades ago and facilitated extensive characterization and knowledge of how to grow cells efficiently for protein or vaccine production. Today, cell lines like CHO produce over 70 percent of the world’s biologic drugs. The cultivated meat ecosystem now has a similar opportunity to leverage these cell lines and media together as “chassis materials” and establish a deeper understanding of how to cost-effectively, sustainably, and safely cultivate cells for food production.”
-MAANASA RAVIKUMAR, PHD, SENIOR SCITECH SPECIALIST, GFI APAC

Living our values, inspiring similar efforts
This first-of-a-kind endeavor would not have been possible without the financial support of GFI’s global donor community, which enabled us to seize this opportunity. For far below market value, we were able to acquire assets that took literally tens of millions of dollars to develop, and in doing so, we can advance the whole cultivated meat field. Among GFI’s core values is sharing information freely—donor support enabled us to do exactly that in an extraordinary way.
Our partnership with Tufts also proved key. Specifically, the Tufts University Center for Cellular Agriculture (TUCCA) is world-renowned for their scientific rigor and research on cultivated meat. They’re also leaders in education, offering the world’s first undergraduate degree in the field. Collaborating with the TUCCA team ensures these cell lines and assets will be in trusted hands and made publicly available in ways that accelerate the entire field.
We are also grateful for our partnership with the Bezos Earth Fund and Bezos Centers for Sustainable Protein in North Carolina, London, and Singapore. Such centers existing at this moment can further accelerate research, develop new technologies, train a skilled workforce, foster collaboration, and ultimately help make cultivated meat nutritious, affordable, and widely available to consumers.
Looking ahead, it is important to note that these bovine cells represent just one species. We will need a similar level of high-quality, openly available cells and media to advance cultivated pork, chicken, fish, and other seafood species, which lag even further behind.
We hope this pioneering effort inspires active companies to compost their cell lines, media, and other tools that they do not plan to take to commercial production. We are also hopeful that companies that have run out of funding follow a similar path as SCiFi Foods, who carefully planned their company wind-down and auction of their assets. These actions can collectively accelerate and democratize cultivated meat research for the greater good.
Please contact philanthropy@gfi.org if you would like to support GFI’s work to advance cultivated meat innovation and catalyze other alternative protein progress.
Please contact maanasar@gfi.org if you are interested in GFI’s cell line projects, banking your cells at a public repository, or adding your cells to our global cell line database.


