
Deep dive: Fermentation metabolism and feedstocks
Explore the diverse feedstocks that fuel microbial production and the processes that convert them to alternative proteins.
Overview of microbial metabolism and fermentation feedstocks
For millennia, microorganisms have transformed plant-derived ingredients into food and beverages through traditional fermentation processes. Bacteria, yeast, and fungi naturally metabolize sugars and other substrates, which can improve the flavors, textures, and nutritional profiles of common plant-based ingredients. This fermentation approach has been used to create many of the food and drink staples we enjoy today, including bread, cheese, yogurt, beer, wine, and soy sauce. In biology, the term “fermentation” often refers to anaerobic respiration, a metabolic pathway that enables organisms to generate energy in the absence of oxygen. However, in the broader context of biotechnology, ‘fermentation’ encompasses a wide range of processes that harness the metabolic capabilities of microorganisms, extending beyond energy generation. This deep dive will discuss fermentation in the broader sense of the word, but when talking about fermentation as an anaerobic metabolism, it’s referred to as ‘metabolic fermentation.’
Modern biotechnology has expanded the applications of microbial fermentation, particularly in the production of alternative protein (AP) products. This includes both biomass fermentation and precision fermentation. Biomass fermentation focuses on growing microorganisms in bulk to harvest all their cellular contents as a protein source. This approach uses carbon feedstocks to drive the production of protein-rich microbial biomass, which can serve as the basis for AP-based meats and other food products. For example, fungi-based proteins like mycoprotein have gained popularity as an animal meat replacement due to their favorable nutritional profile and texture (Linder 2024). Precision fermentation leverages the diverse metabolic products of the microorganisms to produce specific functional ingredients such as proteins, fats, or vitamins, with carbon sources fueling microbial growth and biosynthesis. By engineering microorganisms to express specific genes, scientists can sustainably produce dairy proteins, such as caseins and whey, or egg proteins, like ovalbumin, without the need for animals (Eastham and Leman 2024).
All cells require energy, carbon, nitrogen, and other essential nutrients to grow. In a fermentation bioprocess, feedstocks generally refer to the predominant raw materials used as a source of carbon, nitrogen, and energy for microorganisms to grow and produce edible microbial biomass or precision protein ingredients. The right feedstocks must be paired with the appropriate microorganisms to achieve the desired end product, as this can significantly impact the efficiency of the fermentation process, the quality of the final product, and both the cost and sustainability of the production system.
Sugar sources are the basis for the majority of traditional bioprocesses, as these low-cost sugar substrates, derived from sugarcane or corn starch, can be easily and efficiently metabolized by microorganisms like yeast or fungi, yielding high product yields (Lips 2022). However, there is growing concern for the long-term sustainability of these practices as they contribute to land use and compete with food supplies. This has led to increased interest in exploring more sustainable feedstock alternatives such as complex sugar byproducts from the agroindustrial sector (Bajić et al. 2022) or biogas fermentation (Pieja, Morse, and Cal 2017; Sillman et al. 2019). As we transition to these more sustainable options, understanding how microorganisms metabolize diverse feedstocks will be essential for optimizing fermentation processes and ensuring the efficiency and environmental viability of AP production.
Ultimately, the ability to harness and manipulate microbial metabolism is driving a revolution in food production, allowing scientists to develop animal-free protein that can be biomanufactured to meet the growing global demand for nutritious and more sustainable food sources. This deep dive will give an overview of microbial metabolism and discuss the strategies microorganisms use to convert diverse feedstocks into high-protein biomass and AP-relevant products.
What is microbial metabolism?
Microbial metabolism refers to the intricate network of biochemical reactions that microorganisms use to process nutrients and create energy to sustain themselves. It consists of a series of interconnected pathways that either build or break down complex molecules. Anabolism is the term used for biosynthetic pathways that convert simple molecules into complex macromolecules, such as proteins, nucleic acids, and lipids. Anabolic processes require input of cellular energy (i.e., adenosine triphosphate (ATP)). In contrast, catabolism encompasses the biochemical pathways that break down complex molecules into simpler forms and release energy. This energy is typically harnessed by the cell to fuel other anabolic processes. These networks vary across microbes, leading to diverse pathways supporting microbial life.
Both anabolism and catabolism are essential to maintaining cellular balance and allowing microorganisms to grow and reproduce. The interplay between these pathways allows microorganisms to efficiently utilize available feedstocks and turn them into desired AP-relevant products. Microorganisms have evolved a wide range of strategies to convert diverse substrates into biomass, proteins, and other metabolites. Some can thrive on sugar feedstocks, while others can create their own sugar and generate biomass from C1 gases, such as CO2. Additionally, physical variables such as temperature and light exposure, or chemical variables such as pH and carbon/nutrient availability, can significantly impact microbial growth rate, metabolic activity, and overall productivity. This allows for a lot of flexibility in selecting and optimizing microorganisms and conditions for AP production needs, whether it be maximizing protein yield, improving nutritional content, or improving sustainability. By understanding and leveraging the unique metabolic capabilities of different microbes and the conditions under which they operate best, it is possible to fine-tune production systems and meet the specific needs of AP production.
Microbial nutrient needs
Macronutrients
All microorganisms require essential macronutrients to support growth and metabolic function. Macronutrients are nutrients that organisms typically need in large amounts. The essential elemental macronutrients for life are carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur. In addition to these nutrients, all microorganisms must generate energy to drive metabolic processes and maintain balance in their chemical reactions (also known as reducing power for redox balance). All these components are considered essential for life as cells cannot be assembled or function without them (Merchant and Helmann 2012).
- Carbon (C) is the most abundant constituent element in many microorganisms and is the backbone for organic molecules like proteins, carbohydrates, lipids, and nucleic acids. Carbon can come from a wide variety of sources, including sugars, organic acids, and C1 compounds like CO2 and methane (see carbon feedstocks section below).
- Hydrogen (H) and Oxygen (O) are the basis of water (H2O), which constitutes ~70% of a microbial cell. While some organisms (i.e., anaerobes) might not require molecular O2, all microbial metabolism is based on aqueous chemistry, which facilitates the dissolution and movement of substrates and the transportation of ions for metabolic processes.
- Nitrogen (N) is essential for the synthesis of amino acids, nucleic acids, and other cellular components. Nitrogen can be sourced from both synthetic and biogenic processes (see nitrogen feedstocks section below).
- Phosphorus (P) is essential for energy transfer within the cell. It is a main component of ATP (adenosine triphosphate), which stores energy to complete metabolic processes. It is also an important component of nucleic acids, which hold the microorganism’s genetic information (DNA or RNA). Phosphorus is also contained within phospholipid molecules that form the membranes protecting the cell.
- Sulfur (S) is a central component of sulfur-containing amino acids methionine and cysteine, which are important for the structure and function of proteins by forming disulfide bonds, which stabilize protein structure.
- Reducing power, often in the form of NADH or NADPH, is essential for biosynthesis and maintaining cellular redox balance. NADH is primarily used in catabolic reactions where it donates electrons to the electron transport chain or other substrates to generate ATP. NADPH is typically used in anabolic reactions where it provides reducing power for the synthesis of macromolecules, including amino acids, nucleic acids, and lipids.
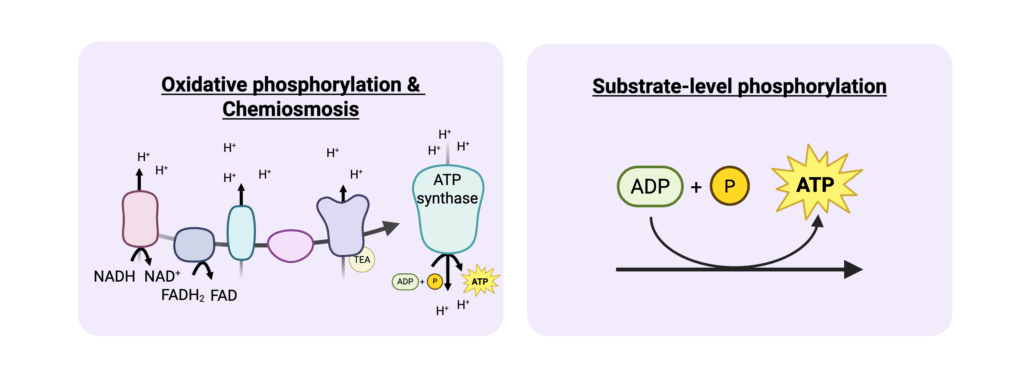
- Adenosine triphosphate (ATP) is the primary energy currency of the cell that is used to power nearly all cellular processes. It is produced when adenosine diphosphate (ADP) is phosphorylated. While ATP is chemically the same across all organisms, how it is produced and the efficiency of those processes vary significantly depending on the organism’s environment and metabolic capabilities. ATP is generated through two main mechanisms: oxidative phosphorylation and substrate-level phosphorylation.
- In oxidative phosphorylation, ATP is produced using an electron transport chain where electrons (either from reducing equivalents like NADH, PQQH2, and FADH or other electron donors) are passed through a series of protein complexes (electron transport chain) which generates a proton (H+) gradient that drives ATP synthesis (Figure 1). In aerobic metabolism, the terminal electron acceptor is O2, whereas anaerobic microbes can use different electron acceptors such as nitrate, sulfate, or carbon dioxide. This process is highly efficient and generates a large amount of ATP through chemiosmosis (typically H+ movement down an electrochemical gradient to produce potential energy).
- Substrate-level phosphorylation generates ATP by transferring a phosphate group from a high-energy substrate to adenosine diphosphate (ADP) (Figure 1). Examples of this are found in glycolysis and the tricarboxylic acid cycle and do not use an electron transport chain. Substrate-level phosphorylation yields much less ATP than oxidative phosphorylation and chemiosmosis.
Micronutrients
Micronutrients are nutrients that organisms typically need in trace amounts. Microorganisms require a range of micronutrients for enzymatic functions, structural components, and metabolic processes. The minimum requirements for micronutrients are less established; however, all cells likely require zinc, magnesium, and iron (Merchant and Helmann 2012).
- Zinc (Zn) plays an integral role in microbial metabolism as a cofactor for over 300 enzymes and contributes to protein stabilization and folding (Coleman 1992).
- Magnesium (Mg2+) is considered a major biological cation that helps stabilize membranes and acts as a cofactor in many enzymatic reactions (Groisman et al. 2013).
- Iron (Fe) is a key component of many enzymes involved in electron transport and redox reactions, such as cytochromes and Fe-S clusters. The transfer of electrons and coupling of redox reactions is what allows microorganisms to metabolize substrates, generate ATP, and carry out biochemical processes necessary for growth and production.
Other micronutrients that are required for most cells include potassium, calcium, cobalt, copper, manganese, and molybdenum. A thorough review of their importance in cellular function can be found in (Merchant and Helmann 2012).
Carbon and energy sources
While metabolism can vary drastically across different microbes, all living organisms need energy and carbon. Energy generation is a critical aspect of microbial metabolism, which facilitates the synthesis of cellular components and overall cellular function. Carbon is an essential building block for all living organisms, forming the backbone of organic molecules such as proteins, lipids, carbohydrates, and nucleic acids. Feedstocks provide these necessary energy and carbon sources and can be converted by the microorganism into protein-rich microbial biomass or other valuable bioproducts. The type of feedstock chosen depends on the metabolic capabilities of the microorganism and is crucial for optimizing microbial growth and productivity.
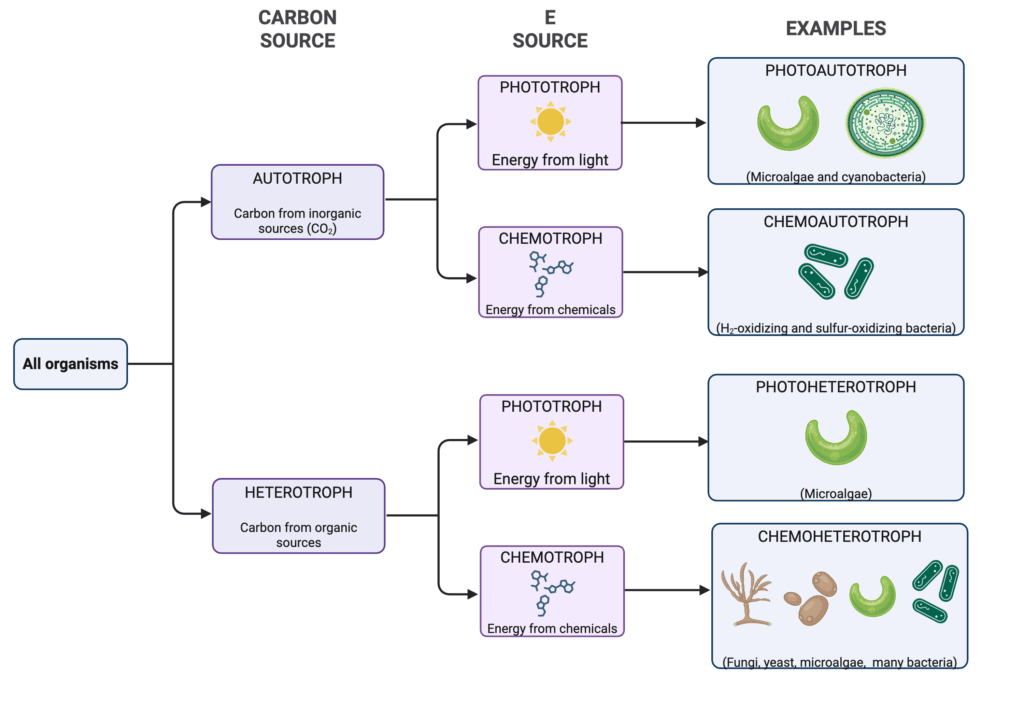
Microorganisms are classified based on both the energy source and the carbon source they use for metabolism (Figure 2). They are classified as phototrophs or chemotrophs depending on the energy source they use to incorporate carbon into their cells. Phototrophs use light energy, making them suitable for growth in photobioreactors and requiring ample access to light (Wada, Vincent, and Mackey 2022). Chemotrophs derive energy from the oxidation of organic compounds, such as glucose, methane, and methanol, or inorganic compounds, such as hydrogen gas (H2), hydrogen sulfide, or ammonia.
Furthermore, microorganisms are classified as autotrophic or heterotrophic depending on the carbon source they use (Figure 2). Autotrophs obtain their carbon from inorganic sources (mainly CO2), which can be sourced from industrial emissions (Marcellin et al. 2022) or direct air capture (Linder 2019; Ruuskanen et al. 2021; Sillman et al. 2019). Heterotrophs obtain their carbon from a wide variety of organic sources, such as sugars derived from sugarcane and corn crops (Lips 2022), agricultural waste and food industry byproducts (Bajić et al. 2022), or carbon capture and utilization (Van Peteghem et al. 2022).
Some microorganisms, including many microalgae and hydrogen-oxidizing bacteria (HOB), are capable of mixotrophy, meaning they can utilize both inorganic and organic carbon sources. This versatile metabolism allows mixotrophs to adapt to varying feedstock availability and can be used to boost yields and productivities for biomass and metabolite production (Castillo et al. 2021; Z. Zhang et al. 2017). The overall diversity of microbial metabolism allows microorganisms to convert a wide range of feedstocks into valuable products, making them versatile agents in biotechnological applications for AP production.
Nitrogen sources
Nitrogen is one of the most crucial elements in the synthesis of microbial proteins. It is an integral component of amino acids and nucleic acids, the building blocks of proteins and DNA/RNA, respectively. While it is the most abundant element in Earth’s atmosphere (~78 percent), atmospheric nitrogen (N2) is not considered bioavailable for most living organisms. Instead, N2 must be converted into a bioavailable form such as ammonia (NH3), nitrate, or nitrite through N2 fixation. This is most commonly achieved biologically in nature through microbial N2 fixation, facilitated by metalloenzymes known as nitrogenases. Industrially, it is most commonly accomplished using the Haber-Bosch process, which reacts N2 with H2 to generate NH3.
The Haber-Bosch process revolutionized farming in the early 1900s by enabling the mass production of nitrogen fertilizers, which greatly increased agricultural productivity. However, this process is energy-intensive (Matassa et al. 2015) and contributes significantly to anthropogenic CO2 emissions (Bicer et al. 2017; Hasler et al. 2015; S. Zhang et al. 2019). More sustainable alternatives are being considered, such as green ammonia (Mayer et al. 2023), fermentation using a consortium of N2-fixing hydrogenotrophic bacteria (Hu et al. 2020), or recovery of NH3 from wastewaters (Matassa et al. 2015; Xiang et al. 2020). However, inorganic nitrogen, in the form of ammonia and ammonium ions, is currently preferred due to their cost, availability, and ease of integration into fermentation bioprocesses, due to their high solubility.
Biogenic nitrogen sources, such as amines and amino acids, can also be used in microbial fermentation. These compounds are typically found in protein hydrolysates, peptones, and industrial byproducts, such as yeast extracts, molasses, and corn steep liquor. The nitrogen feedstocks are not only sourced more sustainably than synthetic feedstocks, but they also require less cellular energy to convert into usable forms (see nitrogen feedstocks).
Microorganisms used in alternative protein production
One of the major factors impacting the efficiency, scalability, and nutritional quality of alternative protein products is the microorganism. Each type of organism–bacteria, yeast, fungi, and algae–offers distinct metabolic capabilities that can influence the production process. Microorganism selection is typically based on a variety of factors, including, but not limited to, their ability to metabolize specific substrates, growth rate, protein production profiles, genetic tractability, and end product composition.
Bacteria
Bacteria are highly versatile microorganisms, capable of rapid growth and diverse feedstock utilization. Some can grow autotrophically using inorganic carbon sources (e.g., CO2 and CO), such as cyanobacteria and hydrogen-oxidizing bacteria. Most bacteria grow heterotrophically using organic carbon sources (e.g,. sugars, organic acids, and CH4). They can also derive energy from either light or the oxidation of various compounds, making them suitable for phototrophic or chemotrophic growth (Figure 2).
Bacteria have long been used in traditional food fermentation to metabolize feedstocks in food (such as sugars, proteins, and fats) to produce compounds that enhance the flavor, aroma, and preservation of foods. For instance, lactic acid bacteria (LAB) like lactobacillus species are used in yogurt, sauerkraut, and kimchi to ferment sugars into lactic acid. While traditional fermentation typically relies on low concentrations of bacteria, modern approaches like biomass and precision fermentation utilize bacteria at much higher concentrations. On average, bacteria are made up of about 50-65 percent protein (dry weight; (Y.P. Li et al. 2024) and can grow to high concentrations in <48 hours under optimal conditions (Eastham and Leman 2024), providing a rapid and efficient means of producing protein-rich biomass or ingredients. Additionally, the well-characterized genetic systems and low endotoxin production of many Gram-positive bacterial strains, including B. subtilis, C. glutamicum, and L. lactis, can facilitate both the optimization of feedstock usage through targeted genetic engineering (Zhang et al. 2022) and the production of food-grade products through precision fermentation (Eastham and Leman 2024). The applications for bacteria-derived protein are still emerging, with opportunities in alternative meat, dairy, and high-protein flours. Commercially available food products derived from bacterial fermentation include Solein®, Air Protein®, UniProtein®, Positive Protein, and SuperBrewed Protein.
Fungi
Fungi, which include both yeasts and filamentous fungi, are increasingly being considered for use in AP production. They are exclusively chemoheterotrophs, relying on organic substrates, such as sugar, to grow. Yeast have a protein content of 45-55 percent dry matter and filamentous fungi consist of about 30-45 percent protein (Y.P. Li et al. 2024), making them good candidates for producing edible high-protein biomass called mycoprotein. Yeast have the added benefit of being widely accepted due to their long use in traditional fermentation. Moreover, filamentous fungi, such as Trichoderma reesei and Aspergillus niger, are known to produce enzymes that aid in the breakdown of cellulosic materials, allowing them to valorize agricultural side streams such as wood and non-food crops (Dashtban, Schraft, and Qin 2009). Given that fungi are eukaryotes, they perform similar post-translational modifications (PTMs) to animals, making precision fermentation easier in these organisms (Eastham and Leman 2024). Fungal-derived products from Fusarium (e.g., QuornTM, ABUNDA®, Fy ProteinTM), Saccharomyces (e.g,. nutritional yeast, Marmite, and Vegemite), and Candida (SylPro® and Yusto®) are commercially available. Other commercially available fungi-derived protein products include Fermotein®, MeatiTM, MyBacon®, and FermentIQTM.
Microalgae
Microalgae are single-celled organisms that can be broadly classified as green algae (Chlorophyta), red algae (Rhodophyta), or brown algae (Phaeophyta). They are photoautotrophic organisms that utilize light energy to fix CO2 and produce glucose for metabolism. These organisms can also exhibit mixotrophic growth under certain conditions, allowing them to metabolize organic substrates alongside inorganic carbon sources, which can boost growth and biomass production rate and allow for variable feedstock usage (Bajić et al. 2022).
The protein content of microalgae is largely dependent on the species, but is typically high in the range of 40-60 percent (dry weight) (Y.P. Li et al. 2024) and has an amino acid profile that is similar to other food proteins (Gouveia et al. 2008), making them powerful tools for high-yield protein production. In addition to proteins, microalgae are rich in other nutrients, including fats, vitamins A, B, C, and E, chlorophylls, and carotenoids (Gouveia et al. 2008). However, digestibility and protein extraction can be difficult due to the rigid cell wall structure (Yang et al. 2024). Consumer acceptance, specifically concerning organoleptic properties (e.g. color, taste, and texture), is an important factor to consider in scaling up the use of microalgae for AP production (Yang et al. 2024).
Commercial applications of algae for protein products are increasing in popularity. For example, MEYTM is a microalgae-derived AP product produced by Solmeyea that is being sold for human consumption in Europe. Triton Algae Innovations has successfully harnessed green algae, specifically Chlamydomonas reinhardtii, to create algae-based alternative proteins, as well as using red algae to develop alternative meat ingredients. Finally, the Israel-based company Brevel is leveraging the unique ability of mixotrophic microalgae to use a variety of carbon and energy sources and creating cost-competitive microalgae-based AP ingredients.
Carbon feedstocks for microbial metabolism
The choice of carbon feedstocks is often what drives the efficiency and sustainability of microbial fermentation processes for AP production. Ultimately, the choice of feedstock and optimization of microbial metabolism must align with the process goal (Noroozi and Jarboe 2023). There are many different feedstocks, each with its own benefits, challenges, and metabolic implications for the fermentation process, some of which are outlined in Table 1.
| Carbon feedstock | Sources | Preprocessing | Metabolism |
|---|---|---|---|
| 1st Generation Sugars | |||
| Glucose, Sucrose, Fructose | Food crops (e.g., sugarcane, sugar beets, fruit, honey) | Milling, refining, and extraction of pure sugar | Glycolysis → fermentation/respiration |
| Starch | Food crops (e.g., corn and potatoes) | Hydrolyzed to glucose | Glycolysis → fermentation/respiration |
| 2nd Generation Sugars | |||
| Lignocellulosics | Agricultural residues (e.g., bagasse, corn stover, and wheat straw) Forestry waste Non-food crops (e.g., grasses) | Hydrolysis to release glucose, xylose, and other sugars | Glycolysis/PPP → fermentation/respiration |
| 3rd/next Generation Sugars | |||
| Algae | Microalgae and macroalgae | Enzymatic or chemical hydrolysis to release fermentable sugars like glucose | Glycolysis → fermentation/respiration |
| Complex sugar mixtures | Food waste (e.g., fruit and vegetable peels, bread, dairy) Industrial byproducts (e.g., molasses, brewery spent grains) | Enzymatic or chemical hydrolysis to release fermentable sugars | Glycolysis → fermentation/respiration |
| Inorganic C1 | |||
| CO2 | Direct air capture Flue gas | None | Carbon fixation pathways (CBB cycle, Wood-Ljungdahl pathway, rTCA cycle) |
| CO | Industrial off-gas Syngas CO2 | Reverse water-gas shift reaction of CO2 | Conversion to CO2 using CODH and carbon fixation via CBB cycle or Wood-Ljungdahl pathway |
| Organic C1 | |||
| Methane | Natural gas Biogas from anaerobic digestion | None | Methanotrophy (RuMP cycle, Serine cycle, CBB cycle) |
| Methanol | CH4 CO2 | Methane reformation or electrochemical/ photochemical CO2 reduction | Methylotrophy (bacterial RuMP/yeast XuMP cycles, rGly pathway, Serine cycle, CBB cycle) |
| Formate | CO2 | Electrochemical/ photochemical CO2 reduction | rGly pathway and Serine cycle |
| Organic C2 | |||
| Acetate | CO2 Acetogenesis Wood distillates | Electrochemical reduction of CO2 or wood pyrolysis | Acetotrophy (direct precursor to acetyl-CoA for biosynthesis) |
Sugars: C5 and C6 substrates
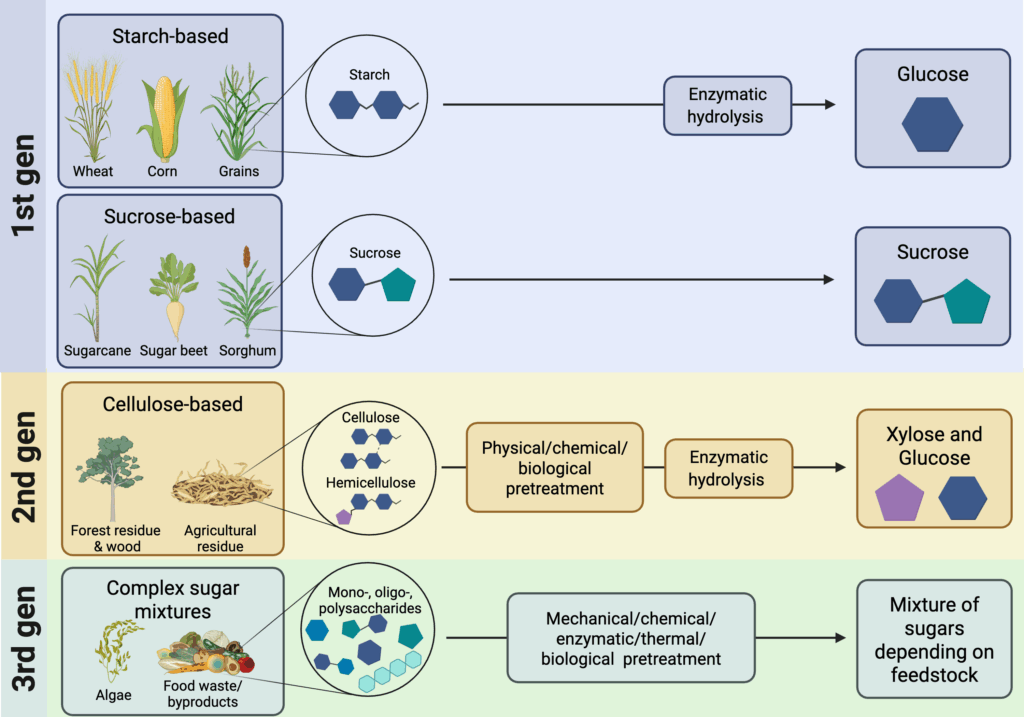
Sugars are among the most widely used feedstocks for microbial fermentation in food production, pharmaceuticals, biofuels, and other chemicals. Sugars such as glucose, sucrose, and xylose serve as primary organic carbon sources in a variety of heterotrophic metabolisms and are used to fuel cellular growth and product synthesis. Sugar feedstocks can be categorized as first-generation, second-generation, or third-generation sugars based on their source and processing methods (Lips 2022).
First-generation sugar feedstocks
First-generation sugars are derived from food crops such as sugarcane, sugar beets, and corn. With minimal processing, these sugar- and starch-rich crops can be converted into “simple” mono- and disaccharide sugars (e.g., sucrose and glucose) (Figure 3). Glucose can be directly used by heterotrophic microorganisms as a carbon source. Organisms containing an invertase (e.g., S. cerevisiae, Aspergillus spp, and Bacillus spp.) can break down sucrose into glucose and fructose for use. First-generation sugars are reliable because they are already widely cultivated and have established supply chains. They also have low production costs that ranged between $0.21-0.55 per kg sugar in 2017 (Cheng et al. 2019) due to minimal processing requirements.
While sugar-fed fermentation for alternative protein is expected to make an increasingly large impact on the sustainability of food production upon continued scale up (Humpenöder et al. 2022), the sustainability of the system could be further increased by avoiding the intensive sugar refining steps associated with corn and sugarcane sugars (Taylor et al. 2023). The production of sugar-fed microbial protein has been modeled to be highly environmentally advantageous over ruminant meat production (Humpenöder et al. 2022). Currently, low-cost and easily processed sugar substrates fuel a significant portion of the industrial fermentation sector, including AP production (Lips 2022).
Second-generation sugar feedstocks
Second-generation sugars are considered more sustainable alternatives to first-generation sugars, as they are derived from renewable lignocellulosic sources such as wood, non-edible agricultural residues (e.g., bagasse, corn stover, and wheat straw), and non-food crops (e.g. switchgrass). Lignocellulosic biomass is considered the world’s most abundant renewable resource (Kiefer et al. 2021) and is rich in complex carbohydrates, including cellulose (30-50 percent), hemicellulose (15-30 percent), and lignin (10-25 percent) (Lips 2022). However, these biomass carbon sources require extensive pretreatment to extract and hydrolyse into fermentable sugars, resulting in production costs that ranged between $0.11-3.37 per kg sugar in 2017, at most nearly 15 times more than those of first-generation sugars (Cheng et al. 2019).
The lignocellulose structure is considered highly stable and recalcitrant to processing into sugars. It is typically disrupted via physical, chemical, or biological processes to make the cellulose and hemicellulose accessible for subsequent enzymatic hydrolysis into a mixture of fermentable pentose and hexose sugars such as xylose, mannose, galactose, and glucose (Figure 3). While these simple sugar products are easily fermented, the presence of preferred sugar substrates (e.g., glucose) will often prevent the uptake of less preferred ones (e.g., xylose) (D’Amore, Russell, and Stewart 1989; Görke and Stülke 2008). This phenomenon, known as carbon catabolite repression, can limit efficient utilization of mixed sugars such as those derived from lignocellulosic biomass. Work is being done to improve the co-utilization of sugars through synthetic engineering of genetically tractable organisms like E. coli (Nichols, Dien, and Bothast 2001; Trinh, Unrean, and Srienc 2008) and S. cerevisiae (Hou et al. 2017; M. Gao, Ploessl, and Shao 2018) or native xylose-assimilating microbes like O. polymorpha (J. Gao et al. 2023).
While lignocellulosic biomass is a more sustainable source of sugar that does not compete with food supply, complex pretreatment and inefficient substrate utilization present significant challenges for the widespread use of lignocellulosic sugars in industrial fermentation. However, a few companies are working to valorize these feedstocks for AP production. Currently, Arbiom has developed an industrial-scale process to produce protein ingredients for feed and food products using second-generation yeast feedstocks, such as agricultural byproducts and wood residues. Another company, Enifer Bio, is leveraging fungi to valorize agrifood industry byproducts into mycoprotein ingredients.
Next generation sugar feedstocks
So-called “third-generation” and “next-generation” sugar feedstocks usually consist of complex mixtures of sugars and carbohydrates. They can be derived from algal biomass (Lips 2022) or various waste streams, including those from the food industry.
Algae have a high sugar content of 40-70 percent dry matter (Lips 2022), typically including alginate, mannitol, and glucans (Lee et al. 2024). They can achieve a photosynthetic efficiency up to 3-fold higher than terrestrial biomass (Del Río et al. 2020), resulting in higher biomass yields and improved land-use efficiency. Additionally, their cultivation in coastal waters eliminates the need for arable land and their low lignin content results in easier pretreatment compared to lignocellulosic biomass (Yun et al. 2016; Gomes-Dias et al. 2020). However, these factors along with their high protein content, which typically ranges from six to 43 percent dry matter but can be as high as 80 percent in certain species (Offei et al. 2018; Janssen, Wijffels, and Barbosa 2022), make them promising candidates as a direct protein source rather than a feedstock for sugar extraction (Barbosa et al. 2023). Thus, algal biomass is less explored as a feedstock, with greater focus on its use as protein-rich biomass for food production. As algae-derived protein production scales up, use of algal sugars as feedstocks for other bioprocesses is a co-production opportunity. Thus, algae remain a promising potential feedstock source for future biomanufacturing of fermentation-derived alternative proteins.
Food industry wastes and byproducts represent untapped sources of organic matter and sugars that can be processed into sugar feedstocks for microbial fermentation. For example, molasses, a byproduct of sugar cane and sugar beet processing, contains fermentable sugars (e.g. sucrose, glucose, and fructose) as well as micronutrients, which make it a potential substrate for microbial fermentation. Other examples include fruit and vegetable waste, which are rich in sugars like glucose and fructose, and brewery and winery byproducts, which can provide sugars and organic compounds for use in microbial fermentation. While currently underutilized, there is a large body of work studying food waste as a substrate for a range of microorganisms, including yeast, fungi, algae, and bacteria (Salazar-López et al. 2022). By valorizing these underutilized substrates, the food industry can contribute to a more sustainable and circular bioeconomy by converting waste into valuable inputs for AP products.
Companies are increasingly considering next-generation wastestream feedstocks to improve sthe ustainability of food and bioproduct production. For instance, Sophie’s Bionutrients is looking to repurpose local food waste from breweries, tofu makers, and sugar refineries to produce microalgal protein in urban areas. Although not making food products themselves, the US-based startup, Hyfé, is working to valorize wastewater from food and beverage processing industries by transforming it into low-cost alternative sugar feedstocks for other biomanufacturers across the fuel, food, and chemicals industries (Schoen and Ruiz 2023).
Sugar metabolism
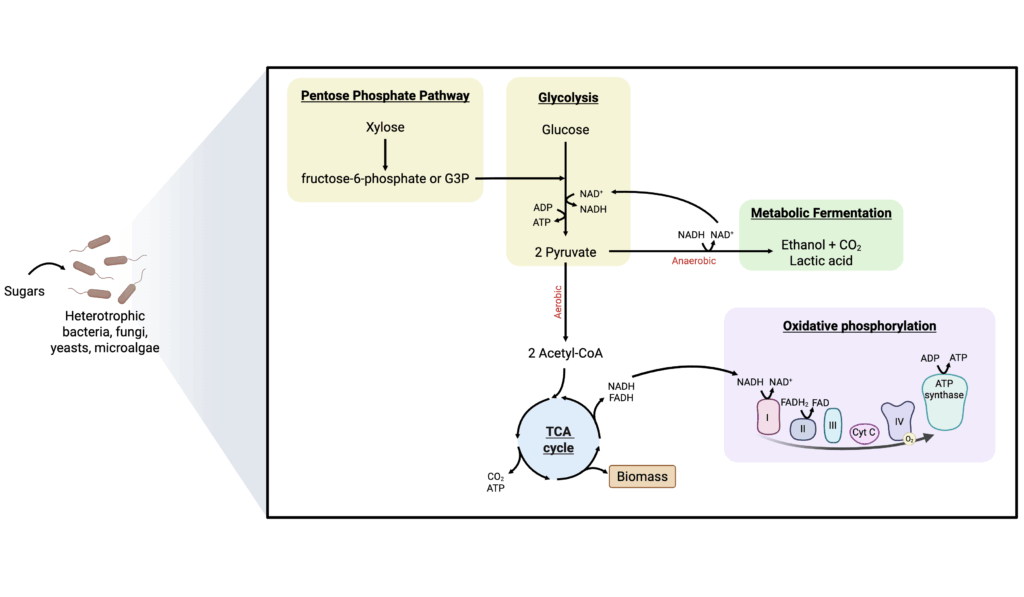
Once the sugar feedstocks have been processed into simple sugars either biotically or abiotically, they can be used as a carbon source by heterotrophic microorganisms to generate a wide-variety of AP products ranging from edible microbial biomass and mycoproteins (Marsh 1985; Pattillo 2021) to recombinant proteins like albumin, caseins, and whey (Pandya et al. 2020). The primary pathway used for simple sugar metabolism is glycolysis (also referred to as the Embden-Meyerhof-Parnas (EMP) pathway), and it is nearly ubiquitous amongst organisms alive today. Its function is to convert a six-carbon glucose molecule into two three-carbon pyruvate molecules through a series of chemical reactions. Glycolysis generates ATP through substrate-level phosphorylation, which provides energy for cellular activities, and also produces key intermediates, such as NADH, for reducing power in oxidative phosphorylation (Figure 4). Other hexoses (i.e., fructose and mannose) and pentoses (i.e., xylose) can be utilized by first being converted them into glycolytic intermediates such as fructose-6-phosphate and glyceraldehyde-3-phosphate (G3P), which can feed directly into glycolysis. Pentoses, or five-carbon sugar molecules, are often processed through a parallel pathway called the pentose phosphate pathway (PPP) (Figure 4).
The product of glycolysis, pyruvate, can be metabolized through various pathways depending on the organism, the condition, and the desired end product. For instance, in the absence of oxygen, yeast will channel pyruvate into anaerobic respiration pathways, known as metabolic fermentation, to produce ethanol and CO2. This is a key process in traditional brewing, where Saccharomyces yeast strains are commonly employed to convert malted grains, such as barley, into alcohol. The CO2, along with other metabolic intermediates, including esters and phenols, contributes to the organoleptic properties of beer, namely its fizziness, aroma, and flavor (Pires et al. 2014). Similarly, in the AP-production industry, lactic acid bacteria can ferment carbohydrates into lactic acid while producing metabolites that contribute to the flavor, nutrition, and reduced allergenicity of certain protein products (Wang et al. 2021).
In the presence of oxygen, yeast perform aerobic respiration where pyruvate gets converted to acetyl-CoA using a pyruvate dehydrogenase complex (PDC). Acetyl-CoA then acts as a substrate for the tricarboxylic acid (TCA) cycle (also referred to as the citric acid cycle or the Krebs cycle), which produces important intermediates used in amino acid synthesis. High amounts of ATP are generated through oxidative phosphorylation, utilizing the electron transport chain and oxygen (O2) as the terminal electron acceptor (Figure 4). This process yields significantly higher amounts of ATP and biomass compared to anaerobic respiration, making it particularly useful for producing high-protein biomass.
Commercial and large-scale considerations for sugar feedstocks
When selecting sugar feedstocks for commercial- or large-scale microbial fermentation, economic feasibility, scalability, and sustainability are key factors. First-generation sugars are popular because they are widely available, cost-effective, and offer high fermentation efficiency due to their purity. However, they potentially face sustainability issues with continued scale-up as they compete with food supply and have a potential resource-use intensivity due to milling and processing. In contrast, second- and third-generation sugars offer more sustainable alternatives, but large-scale use is limited by a lack of consistency and quality, requiring additional processing steps and increasing production costs. To make alternative sugar feedstocks more competitive at a commercial scale, research and technological advancements have focused on improving the efficiency of their conversion into fermentable sugars while reducing preprocessing costs. One promising approach is strain development, where microorganisms are genetically engineered to utilize recalcitrant or mixed sugar feedstocks directly. This allows more sustainable sources, such as lignocellulosic residues or sugar waste streams, to be efficiently valorized (Balagurunathan et al. 2022).
However, simply improving feedstock utilization is not enough; optimizing the fermentation process itself is crucial to achieve the desired yields and efficiency at a commercial scale. This means optimizing growth conditions to maximize biomass or bioproduct while minimizing feedstock usage. For example, sufficient media aeration and glucose-limited feeding strategies are often employed in yeast fermentation to encourage aerobic respiration and biomass production while reducing metabolic fermentation and ethanol production through the Crabtree effect (Vieira, Andrietta, and Andrietta 2013). The same tactics can be employed for bacteria such as E. coli. These growth conditions become harder to manage during scale-up, where insufficient mixing can result in high-substrate and low-O2 zones, leading to growth-limiting byproduct formation as a result of overflow metabolism (Larsson et al. 1996; Xu et al. 1999).
Ultimately, scaling up sugar-based fermentation processes requires careful consideration of many factors. Efficiently managing these factors and optimizing technologies can help make sugar-based feedstocks for large-scale fermentation-derived AP production economically viable and environmentally sustainable.
One and two-carbon feedstocks
While sugars are well-established and widely used substrates for microbial fermentation, C1 and C2 feedstocks–such as carbon dioxide (CO2), carbon monoxide (CO), methane (CH4), methanol (CH3OH), and acetate (CH3COO)–offer alternative, sustainable routes to convert carbon feedstocks into AP-relevant products. These substrates are classified by the number of carbon atoms in their molecular structure: C1 feedstocks contain a single carbon atom, and C2 feedstocks contain two. Both autotrophic and heterotrophic microorganisms can metabolize these compounds, making them versatile for fermentation processes.
Carbon dioxide feedstocks
CO2 is an inorganic C1 compound that is a promising candidate as a sustainable feedstock for either direct utilization by autotrophic microbes or indirect utilization by other C1 and C2 metabolizing microbes. Direct utilization employs microbes, such as certain algae and bacteria, that can directly convert CO2 into biomass and other products through carbon fixation processes, including the Calvin-Benson-Bassham cycle or the reductive tricarboxylic acid (rTCA) cycle, as discussed below. In contrast, indirect utilization uses microbes that metabolize CO2-derived substrates, such as methanol, formate, or acetate. These compounds are produced via chemical conversions, enabling microbes that cannot directly fix CO2 to benefit from abundant CO2 feedstocks indirectly. CO2 can be sourced from various industrial waste streams or even captured directly from the atmosphere. As of 2023, the global CO2 capture capacity has reached approximately 50 million tons per year (Global CCS Institute 2023), providing a sustainable supply of feedstock for microbial fermentation. Capturing atmospheric carbon, or preventing its release at the point of generation, improves the sustainability of AP production and greenhouse gas emissions by repurposing it into valuable bioproducts.
CO2 is not readily usable for most biological processes, so autotrophic microorganisms, such as cyanobacteria, algae, and certain bacteria, have evolved specialized metabolic pathways to directly capture and convert CO2 into usable forms through carbon fixation. These pathways enable microbes to synthesize organic compounds from inorganic carbon. Energy for these reactions can come from sunlight (in the case of phototrophs, Figure 2) or from the oxidation of inorganic molecules like hydrogen, sulfur, and ammonia or organic molecules like methanol and formate (in the case of chemotrophs, Figure 2). Of the six known carbon fixation pathways (reviewed in Santos Correa et al. 2023), three are most relevant to AP-production and this deep dive: the Calvin-Benson-Bassham (CBB) cycle, the Wood-Ljungdahl pathway, and the reductive tricarboxylic acid (rTCA) cycle (Figure 5).
- Calvin-Benson-Bassham (CBB) cycle: The CBB cycle is the primary carbon fixation pathway in photosynthetic organisms, including algae and cyanobacteria. ATP and NADPH are generated from light energy in the light-dependent reactions of photosynthesis, splitting H2O and passing electrons using its own version of electron transport chain (Figure 5). ATP and NADPH are used in the CBB cycle to convert CO2 into glyceraldehyde-3-phosphate (G3P), a 3-carbon sugar. Two G3P molecules can be enzymatically combined to form one molecule of glucose that can be used as a primary carbon source for cellular growth and biomass production (Figure 5).
- Wood-Ljungdahl pathway: The Wood-Ljungdahl pathway is the most energy-efficient way for microorganisms to assimilate carbon (Fast et al. 2015; Claassens et al. 2016) and is commonly used in anaerobic environments by archaea (e.g. methanogens) and certain bacteria (e.g., acetogens) to convert CO2 into acetyl-CoA. This process typically uses energy from H2 oxidation via hydrogenases, although some microbes can use CO and formate as a source of electrons. Acetyl-CoA then serves as a crucial precursor for biosynthesis, facilitating the transfer of the acetyl group to anabolic processes and pathways that contribute to biomass accumulation (Figure 5).
- Reductive Tricarboxylic Acid (rTCA) cycle: The rTCA cycle, which operates in reverse of the traditional TCA cycle (Figure 4), converts CO2 to acetyl-CoA. The rTCA cycle typically relies on energy from light in phototrophs, or from H2 or sulfur compounds in chemotrophs. Acetyl-CoA then serves as a crucial precursor for biosynthesis (Figure 5).
Several companies are pioneering the use of CO2 as a sustainable feedstock to produce AP products. Air Protein and Solar Foods, for instance, have developed microbial platforms using hydrogenotrophs that can directly metabolize CO2 and H2 to create protein-rich food ingredients with complete nutritional profiles. Similarly, Arkeon Biotechnologies uses CO2 and H2 to feed archaea and produce amino acids for alternative protein ingredients, resulting in a carbon-negative fermentation process. NovoNutrients focuses on using mixed gas emissions containing CO2, H2, and O2 to produce food and feed-grade proteins by employing autotrophic microbes that can fix CO2 into biomass through carbon fixation pathways. Currently at the pilot plant stage, NovoNutrients is refining its looped cylinder bioreactor design to improve gas fermentation energy costs. Other companies, including Arborea, Solmeyea, and Brevel, are leveraging phototrophic carbon fixation in cyanobacteria and microalgae to produce biomass for various food ingredients from CO2, water, and light. Similarly, Ingrediome is using photosynthetic precision fermentation to generate precision fermentation-derived animal proteins from CO2 and light. CO2 can also be chemically converted into other C1 and C2 feedstocks, including carbon monoxide, methane, methanol, formate, and acetate, for microbes that cannot fix carbon directly.
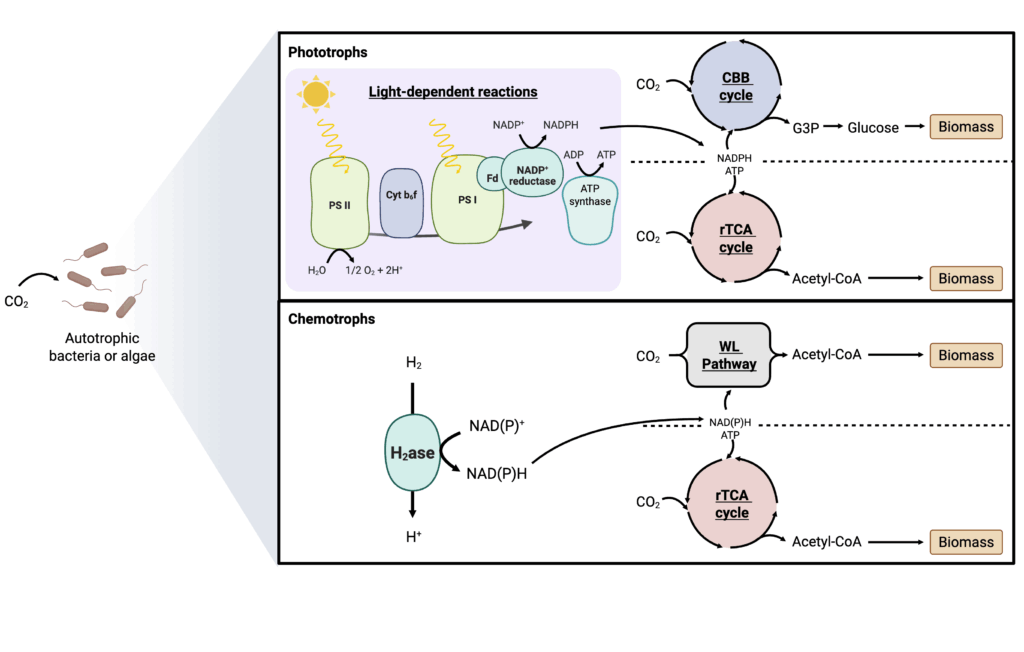
Carbon monoxide feedstock and its metabolism
Carbon monoxide (CO) can be derived from CO2 using the reverse water-gas shift reaction, which reacts CO2 and hydrogen to produce CO and water (Atsbha et al. 2021), with an energetic efficiency of 60-70 percent (Claassens et al. 2019). Additionally, CO is a major component of syngas, a mixture of CO, CO2, and H2 produced through the gasification of carbon-rich materials such as coal, biomass, or waste. However, CO is highly toxic, potentially limiting its use at a commercial scale.
Carboxydotrophic bacteria such as Oligotropha carboxidovorans (formerly: Pseudomonas carboxydovorans) (Meyer 1980) and acetogenic bacteria like Clostridium ljungdahlii (Köpke et al. 2010) can utilize CO as a sole carbon and energy source. CO is converted by the organism into CO2 using carbon monoxide dehydrogenase (CODH), then metabolized aerobically using the CBB cycle to produce G3P or anaerobically using the Wood-Ljungdahl pathway to produce acetyl-CoA (Jiang et al. 2021). Both products can be further converted into biomass (Figure 6).
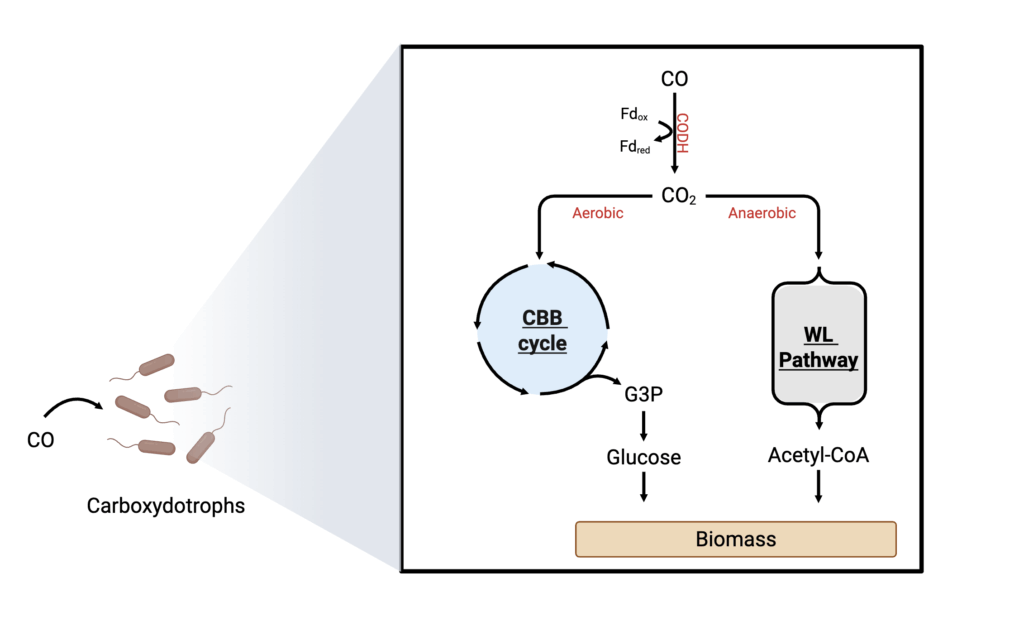
Methane feedstock and its metabolism
Methane (CH4) is an naturally abundant and low-cost feedstock that can be renewably sourced from wastewater treatment plants, landfills, or agricultural wastes as a byproduct of anaerobic digestion, or be renewably generated with an efficiency of 50-60 percent (Claassens et al. 2019) by reacting H2 with CO2 (Götz et al. 2016). Methanotrophs can utilize CH4 as a sole source of carbon and energy in both aerobic and anaerobic metabolisms; however, anaerobic methane fermentation has much lower yields and is less explored (García Martínez et al. 2022).
Methanotrophs oxidize CH4 through a metabolic cascade consisting of four key enzymes: methane monooxygenase (MMO), methanol dehydrogenase (MDH), formaldehyde dehydrogenase (FADH), and formate dehydrogenase (FDH). This process converts CH4 into a series of intermediates, including methanol (CH3OH), formaldehyde (HCHO), and formate (HCOOH) (Sahoo, Goswami, and Das 2021). The intermediates can then be metabolized using various carbon assimilation pathways. Formaldehyde is assimilated using the RuMP cycle, while formate can enter the Serine cycle, both of which produce acetyl-CoA for biosynthesis (Figure 7). Additionally, some methanotrophs from the phylum Verrumicrobia have been found to convert formate to CO2 using formate dehydrogenases (FDH) (Sahoo, Goswami, and Das 2021). The CO2 can then be fixed using the CCB cycle to produce G3P and glucose for biomass generation (Figure 7).
Methylococcus capsulatus has been the primary methanotroph studied to create single-cell protein products (Rasouli et al. 2018; Lieven et al. 2018; But et al. 2024) and is being used at a commercial scale by UniBio and Calysta to create food and feed products. Another company leveraging the use of methane as a feedstock for protein products is String Bio.
Methanol feedstock and its metabolism
Methanol (CH3OH) is traditionally produced from syngas; however, advancements in alternative production methods are expanding the options for more sustainable methanol synthesis (Sepahi and Rahimpour 2023). For example, both methanol and formate (HCOO–) can be derived electrochemically or photochemically from CO2 through hydrogenation reactions (Álvarez et al. 2017).
Methanol and formate feedstocks are particularly attractive because they are miscible, making them easier to handle, transport, and integrate into fermentation systems compared to gaseous C1 feedstocks like H2/CO2 or CH4. Methanol facilitates relatively high mass transfer and microbial productivity but presents challenges such as significant heat production during methanol oxidation, high oxygen requirements, and flammability, all of which can complicate large-scale operation and increase costs (C. Zhang et al. 2022). Formate, on the other hand, is safer to handle due to its lower flammability, but it is more expensive and provides less reducing power due to its more oxidized state (C. Zhang et al. 2022). Aerobic conversion of methanol or formate into biomass tends to have higher energetic efficiencies compared to C1 gasses, such as H2/CO2 and CH4 (Cotton et al. 2020). However, both feedstocks produce formaldehyde as a toxic intermediate during assimilation, which requires careful management in fermentation processes. That said, additional work is needed to determine proper feeding strategies and media preparations to avoid cellular toxicity and promote efficient growth when using methanol and fomate as feedstocks (Cotton et al. 2020).
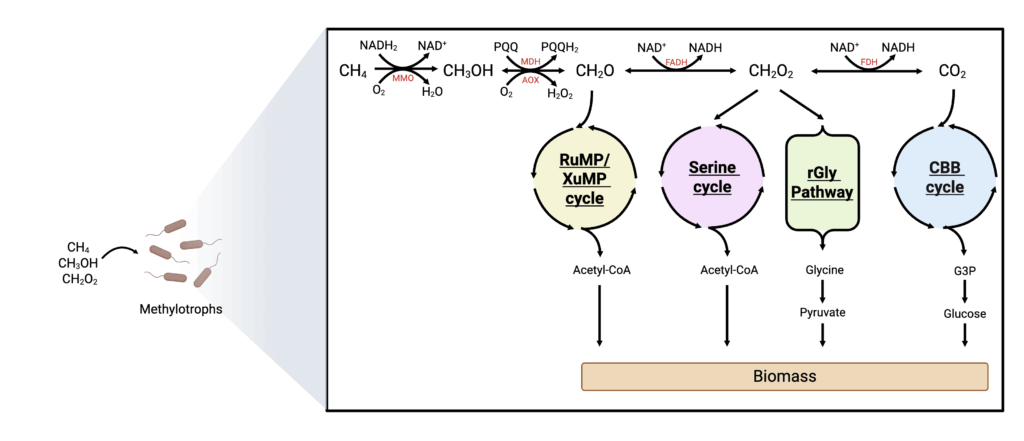
Methanol can be natively metabolized by methylotrophic bacteria (e.g., Bacillus spp.) and yeasts (e.g., Komagaetella phaffi, formerly Pichia pastoris) often using the Ribulose Monophosphate (RuMP)/Xylulose Monophosphate (XuMP) cycles, the reductive Glycine (rGly) pathway, or the Serine cycle to produce biomass (Kelso et al. 2022). The RuMP and XuMP cycles operate using a lot of the same intermediates. In both, methanol is oxidized to formaldehyde, and formaldehyde is fed directly into the cycles to produce G3P, resulting in a fixed carbon source for biomass generation via glycolysis and the TCA cycle (Cotton et al. 2020). A key benefit of organisms using the XuMP cycle is that formaldehyde production from methanol oxidation is confined to the peroxisome, effectively isolating toxic intermediates (Singh et al. 2022). However, the XuMP cycle oxidizes methanol with O2 via alcohol oxidase (AOX) to produce hydrogen peroxide (H2O2), requiring further detoxification, while the RuMP cycle uses NAD+ to store electrons from methanol for other processes, making it more efficient (Kelso et al. 2022). The FDA-approved methylotrophic yeast K. phaffii is a GRAS organism studied for biomass and recombinant alternative protein production via the XuMP cycle (Meng et al. 2023), but it requires improvements in methanol utilization, temperature resistance, and protein synthesis to enhance its competitiveness as an AP production cell factory. The RuMP and XuMP cycles could also potentially support growth on formate if it can be reduced to formaldehyde in vivo, which has been proposed albeit at a very low rate (Cotton et al. 2020).
The rGly pathway was originally proposed as a synthetic route for formate assimilation, but has recently been identified in a few anaerobic species that convert CO2 to formate for carbon assimilation (Kelso et al. 2022). This pathway primarily produces glycine, which can be converted into serine and subsequently pyruvate, feeding directly into central carbon metabolism (Figure 7). Although research on the rGly pathway is still in its early stages, it shows promise for higher biomass yields compared to the RuMP, Serine, and CBB cycles when using formate as a feedstock (Cotton et al. 2020). Its potential has already been demonstrated by engineering the core components of the rGly pathway in E. coli (Kim et al. 2020) and S. cerevisiae (Gonzalez de la Cruz et al. 2019). However, further work must be done to implement methanol utilization via this pathway and improve the conversion of glycine to pyruvate for cell growth (Kelso et al. 2022).
The Serine cycle supports the utilization of both methanol and formate. Methanol can be oxidized to formaldehyde, which can then be converted into formate. Formate can enter the Serine cycle directly, ultimately producing acetyl-CoA for biomass generation (Cotton et al. 2020) (Figure 7). Although methanol assimilation is less energetically efficient than formate assimilation due to the use of quinone-dependent methanol dehydrogenase (MDH) (Claassens et al. 2019), it occurs at a higher rate and is more thoroughly researched.
Methanol and formate are also key feedstocks in anaerobic acetogenic pathways, where they can be directly assimilated into the Wood-Ljungdahl pathway, ultimately producing acetate. This process is promising for specific bioproducts such as acetate (discussed below), organic alcohols, and fatty acids because it avoids the production of formaldehyde as a toxic intermediate and is much more energetically efficient compared to aerobic pathways. The production of AP-relevant products like biomass requires aerobic metabolisms (discussed above), which uncouples biosynthesis from energy conservation, making the process less energy efficient but increasing the biomass yield (Cotton et al. 2020).
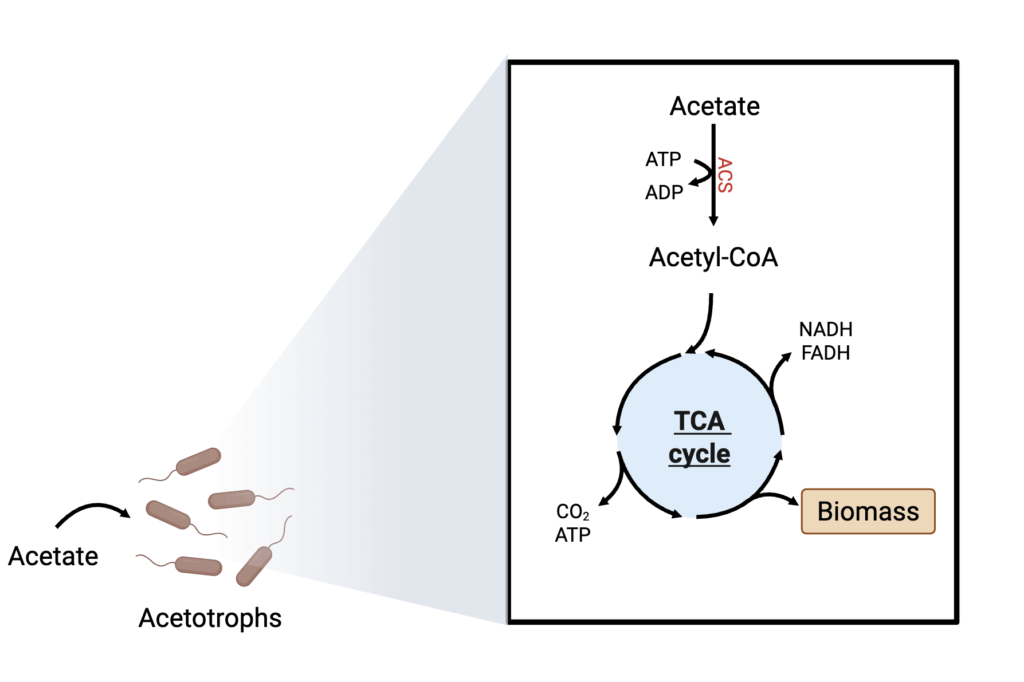
Acetate feedstock and its metabolism
Acetate (CH3COO–) is an emerging and versatile C2 feedstock for microbial fermentation, with growing interest due to its availability and potential sustainability benefits. Acetate can be derived from a variety of sources using biological, photochemical, and electrochemical processes, and then fed to acetate-utilizing microorganisms to produce AP-relevant products like single-cell protein and recombinant proteins (Kiefer et al. 2021). A promising approach is the electrochemical reduction of CO2 to acetate, which leverages renewable energy to power electrochemical systems that convert H2O and CO2 into CO and, subsequently, acetate at a lab efficiency of 25 percent (Hann et al. 2022). Another approach is a multi-step biological conversion process where acetate bioproduction is coupled with acetate bioconsumption to produce valuable bioproducts at a predicted energetic efficiency of up to 26 percent (Claassens et al. 2019; Molitor, Mishra, and Angenent 2019). However, this process still requires optimization to increase production rates and account for increased production costs due to the need for more bioreactors and intermediate processing steps (Vlaeminck et al. 2023). Another plausible source of acetate is through lignocellulosic saccharification, which can produce high amounts of acetate and, as discussed previously, stands as the world’s most abundant renewable resource that does not compete with the food industry (Kiefer et al. 2021).
Like methanol and formate, acetate is a miscible feedstock that facilitates easier transport and handling, as well as increased productivity due to its high solubility and mass transfer properties. While acetate is more costly than methanol and C1 gaseous feedstocks, its global market is rapidly expanding (C. Zhang et al. 2022). It can be fed into bioreactors in pure form and has a lower toxicity compared to methanol and formate, with comparable microbial conversion efficiency (Claassens et al. 2019). Studies even suggest that growth on CO2-derived acetate increased growth rate, protein productivity, and protein yields compared to growth on CO2-derived formate (Sakarika et al. 2020).
Acetate can be directly utilized as a carbon source for a variety of microorganisms, ranging from eukaryotes to bacteria. It is a direct precursor of acetyl-CoA, which is fed into the TCA for energy generation and biosynthesis (Figure 8). Acetate has been explored as a feedstock for various value-added chemicals, including acetone, isobutanol, and isopropanol, among others, especially by E. coli (Kutscha and Pflügl 2020). In the food sector, Triton Algae Innovations has scaled production uses acetate as a carbon feedstock for microalgae-based protein products (Tran et al. 2019; U.S. Food and Drug Administration 2022). Given the high potential of acetate as a sustainable feedstock, the Bill and Melinda Gates Foundation and the Novo Nordisk Foundation have recently funded a consortium that will study AP production using CO2-derived acetate as a feedstock.
Commercial and large-scale considerations for one and two-carbon feedstocks
Overall, C1 and C2 substrates are promising sustainable alternatives to sugar feedstocks due to their lower environmental impact and diverse sourcing options. Gaseous C1 feedstocks such as CO2, CO, and CH4 can be sourced from industrial emissions, biogas from landfills and wastewater treatment plants, or direct air capture systems, and are typically supplied to the microorganisms through a submerged sparger in liquid fermentation. They provide a dual sustainability advantage over refined sugar feedstocks by preventing the global warming potential of C1 gases while also producing sustainable AP products, which reduce the environmental footprint of animal-derived protein products. However, direct utilization of gaseous feedstocks at a commercial scale is currently limited by efficient gas-liquid mass transfer, system cooling, and waste product removal (Silverman, Resnick, and Regitsky 2021).
While indirect CO2 utilization is less commercially explored for food applications, it holds significant potential for sustainable and scalable AP production. By converting CO2 into intermediate compounds such as methanol, formate, or acetate, which can then be metabolized by heterotrophic microbes, this approach can unlock a new pathway for creating protein-rich biomass. Companies are already combining H2 derived from water using renewable electricity and CO2 from industrial flue gases or direct air capture to generate methanol for fuel. With improved purity, similar approaches can be implemented to produce methanol and other CO2-derived compounds as microbial feedstocks sustainably. For example, companies such as LanzaTech have previously scaled gas-fed microbes for commercial production of sustainable fuels and chemicals, and recently announced their intentions to mass produce protein from CO2 (Mridul 2024). Factors such as maximizing substrate utilization efficiency while minimizing toxic intermediates and overheating will be key to optimizing the economic viability of CO2-derived feedstocks at a commercial scale. As companies continue to innovate CO2-based feedstock technologies, both direct and indirect routes hold promise for reducing the environmental impact of protein production while also promoting a circular economy in the food industry.
Nitrogen feedstocks for microbial metabolism
Amino acids are the building blocks of proteins. As the name implies, all amino acids contain an amino group (-NH2), which is derived from nitrogen sources. Amino nitrogen is about 16 percent of the mass of food proteins and thus puts a substantial demand on microbes’ ability to assimilate nitrogen into their biomass (National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances 1989). Microorganisms have evolved ways to metabolize a variety of nitrogen sources, both inorganic and organic. In microbial fermentation, these nitrogen sources can stem from either synthetic (e.g., ammonium salts and urea) or biogenic (e.g. yeast extract, amino acids, and protein hydrolysates) origins. The various nitrogen sources are summarized below.
Synthetic nitrogen
Most synthetic nitrogen sources cannot be directly used for protein synthesis by microorganisms. They must first assimilate the nitrogen into organic forms, primarily amino acids. This assimilation typically involves the uptake of ammonium ions using ammonium transporters, followed by the conversion of those ions into glutamine and glutamate using enzymes such as glutamine synthetase (GS) and glutamate synthase (GOGAT), respectively. These amino acids can then act as nitrogen donors for the synthesis of other amino acids. Ammonium transporters and assimilation enzymes are found in nearly all bacteria, including industrially-relevant E. coli (van Heeswijk et al. 2013) as well as yeasts such as S. cerevisiae (Magasanik 2003).
Ammonium salts such as ammonium sulfate, ammonium chloride, and ammonium nitrate are often supplemented in media formulations as an inorganic nitrogen source. The choice of ammonium salt can vary depending on the specific production needs and the needs of the microorganisms being used. For example, ammonium sulfate is a popularly used nitrogen source because it allows dual delivery of nitrogen and sulfur. This is particularly advantageous for organisms like the commonly used industrial yeast S. cerevisiae, which contain tightly regulated biosynthetic pathways for essential sulfur-containing amino acids like methionine and cysteine (reviewed in Thomas and Surdin-Kerjan 1997). Ammonium chloride is favored for its cost-effectiveness and straightforward nitrogen delivery. Ammonium nitrate also serves as an alternative nitrogen source, but its use is limited due to regulatory concerns related to its explosive nature (U.S. Environmental Protection Agency), which necessitates more stringent handling and storage protocols.
Diammonium phosphate (DAP) is a common inorganic nitrogen source used in fermented beverages (e.g., mead, wine, and cider). It is composed of two ammonium ions and one phosphate ion, making it highly soluble in water. Additionally, DAP provides dual delivery of nitrogen and phosphorus, which may improve productivity compared to other inorganic nitrogen sources in yeast fermentations (Almeida et al. 2020).
Ammonia gas can be sparged directly into the media as a nitrogen source. In aqueous environments, ammonia acts as a proton (H+) acceptor to form ammonium ions (NH4+), which can be easily assimilated by many microorganisms. This reaction also helps to regulate the pH of the culture media by consuming excess H+. This is especially advantageous in bioprocesses involving heterotrophic metabolism, which often produce organic acid byproducts. However, ammonia gas is volatile, particularly at higher pH, and can easily escape from the culture medium without sufficient mixing or pH control, leading to loss of nitrogen.
Ammonium hydroxide (NH₄OH) serves as an alternative nitrogen source with similar pH control benefits, though it, too, can volatilize under alkaline conditions. An added benefit of NH4OH is its ability to absorb CO2, making it especially beneficial as a nitrogen source for microorganisms like microalgae that utilize CO2. In fact, microalgal biomass productivity increases 32.8 percent using NH₄OH as opposed to ammonium nitrate due to increased CO2 uptake (Sun, Sun, and Liu 2022).
Urea, an organic nitrogen source derived from the Haber-Bosch process, can be hydrolyzed into ammonia (NH3) and CO2 in organisms containing a urease enzyme. Urea not only offers the advantage of pH buffering by neutralizing acids, but also provides a controlled and sustained nitrogen release, which reduces the risk of nitrogen overload that can occur with more direct nitrogen sources like ammonia gas or ammonium hydroxide. However, when the goal of the bioprocess is maximizing product formation, the endogenous processing of urea can decrease productivity compared to inorganic and biogenic sources (Zhao, Zhang, and Zhang 2010; Reihani and Khosravi-Darani 2019), perhaps due to less available energy for product synthesis.
These synthetic nitrogen sources, particularly ammonium salts and urea, are often preferred in large-scale fermentation production due to their low cost and high availability. However, other factors should also be considered, such as growth efficiencies and environmental impacts. Converting inorganic nitrogen to amino acids requires more microbial energy and can result in significantly reduced growth rates in yeasts compared to biogenic sources (Albers et al. 1996), ultimately impacting production efficiencies. Additionally, ammonium salts and urea are predominantly derived from the Haber-Bosch process, which is an energy-intensive process that relies on methane, contributing to greenhouse gas emissions (Matassa et al. 2022).
Biogenic nitrogen
Alternatively, biogenic nitrogen sources, including amino acids, peptides, and proteins, can be used to supply a readily available source of nitrogen to the microbes. They are more easily assimilated compared to inorganic sources because they provide nitrogen in a form that is directly usable for protein synthesis. This can improve the efficiency of microbial growth, particularly in processes where rapid biomass accumulation is the goal (Albers et al. 1996; Martínez-Moreno et al. 2012). Additionally, some industrial microbes, including many lactic acid bacteria (Lactobacillus spp. and Streptococcus spp.) (Savijoki, Ingmer, and Varmanen 2006), have incomplete biosynthetic pathways and require exogenous sources of amino acids for growth. By providing the necessary building blocks for protein synthesis, biogenic nitrogen can enhance both growth rates and biomass yields in fermentation processes.
As with carbon feedstocks, there is a sustainability push for nitrogen feedstocks to move away from energy-intensive and unsustainable practices (e.g., the Haber-Bosch process) and towards the valorization of industrial waste streams. This shift is intended to create a more circular economy, where waste and byproducts from one industry serve as valuable inputs for another. Biogenic nitrogen can be derived from more renewable materials such as protein hydrolysates produced from bacterial, plant, or animal biomass–such as yeast extracts, soy peptone, pea peptone, casein hydrolysates, and beef peptone–as well as from industrial byproducts like molasses, and corn steep liquor. Protein-rich raw materials are processed using acid, enzyme, or fungal-assisted hydrolysis (Grossmann 2024). Therefore, the processing costs associated with extracting and purifying these biogenic sources can be higher compared to inorganic sources, impacting their economic feasibility in large-scale processes. Ultimately, balancing cost and sustainability remains a challenge, particularly in large-scale fermentations where a consistent supply of nitrogen is important for process success.
Food and agricultural waste are top candidates for biogenic nitrogen sources because of their high nitrogen and protein content (Grossmann 2024). Of all the major crops produced in North America, soy meal, corn gluten meal, corn distiller’s dried grains with solubles (DDGS), and tomato pomace were the most attractive based on protein content, economic impact, and environmental impact (GFI 2023). Additionally, low-nitrogen waste streams can be supplemented with either biogenic or synthetic nitrogen sources to improve productivity and efficiency (Q. Li et al. 2019; Ouedraogo et al. 2017). This supplementation not only balances out the nutrient profile but also allows the valorization of under-utilized low-nitrogen side streams such as potato or tuber peel waste. Still, work remains to scale up and reduce the cost of efficient sidestream utilization at a commercial scale.
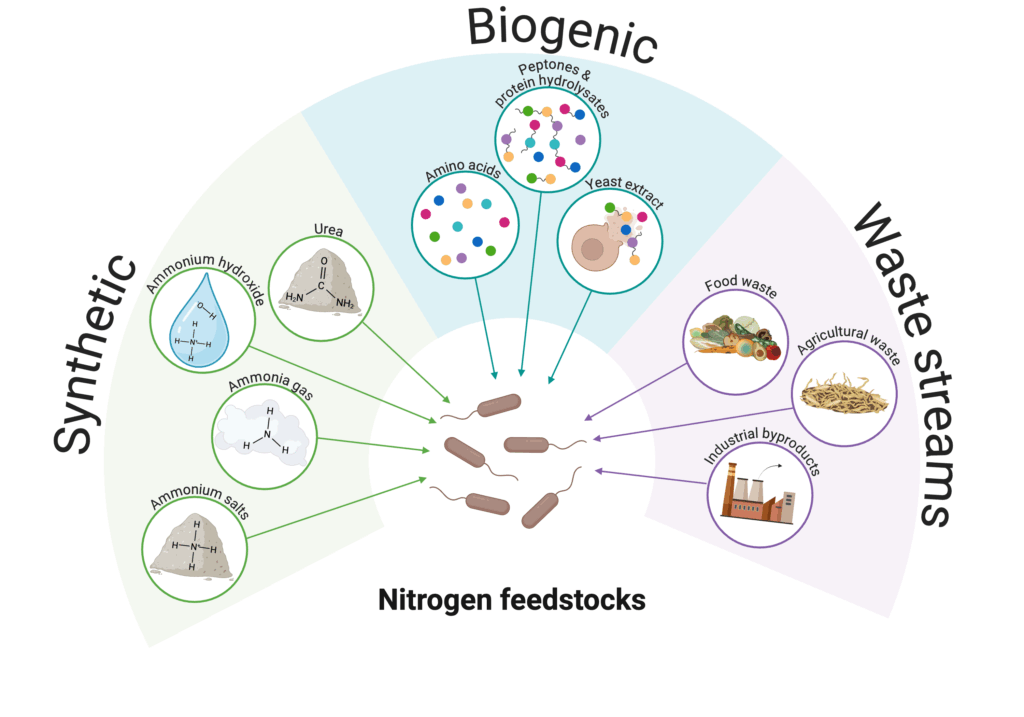
Commercial and large-scale considerations for nitrogen feedstocks
Similar to carbon feedstocks, the choice of nitrogen feedstocks depends on the process goal, as different nitrogen sources can significantly impact microbial growth and productivity, as well as process sustainability and cost (Noroozi and Jarboe 2023). As discussed above, synthetic nitrogen feedstocks, such as ammonium salts and urea, are often preferred due to their low cost, high availability, and high purity, but is produced using energy-intensive processes with high carbon emissions (e.g., Haber-Bosch process). Alternatively, biogenic nitrogen sources from nitrogen-rich agricultural wastes, sidestreams, or fermentation byproducts offer more sustainable alternatives by repurposing waste, though they may be more expensive and variable in composition.
Mixed or co-feedstock approaches—combining two or more different feedstocks for microbial growth—can enhance growth rates and product yields, contributing to cost-effective AP production. For instance, supplementing waste media with glycerol has been shown to improve both biomass yield and protein content of certain industrial yeasts (Kurcz et al. 2018). From a sustainability perspective, integrating waste valorization practices by recycling nitrogen and carbon from waste streams can contribute to reduced emissions and promote a circular economy. While synthetic nitrogen remains cost-effective, the environmental benefits of biogenic sources are increasingly important in reducing the carbon footprint of fermentation-derived AP products.
Regulatory considerations and good manufacturing practices
Ensuring that microbial feedstock processes meet regulatory standards and Good Manufacturing Practices (GMP) is important for the safety, quality, and scalability of AP production. GMP compliance not only ensures worker safety but also ensures product quality and safety, thereby reducing the risk of accidents, equipment damage, and production downtime. However, companies must also factor in the costs of specialized equipment, gas monitoring systems, and safety protocols when designing bioprocesses.
Gas handling safety
In fermentation processes that use gases such as H2, CH4, O2, and CO, strict protocols are required to ensure safe handling and transport. Hydrogen, often used as a source of electrons for hydrogenotrophic metabolisms, and CH4, used as a carbon source for methanotrophs, are highly flammable gases that require proper storage, handling, and monitoring to prevent leaks and explosive risks. Additionally, O2 is often supplied in aerobic fermentation processes to boost microbial growth rates and productivity. While O2 is not flammable, in high concentrations it can increase fire hazard, so GMP protocols require flame-proof equipment, O2 sensors, and proper ventilation to mitigate combustion risks. Carbon monoxide (CO), a toxic and odorless gas, also demands strict air quality control systems as exposure as low as 10 ppm for 8 hours can be harmful to workers (“Air Quality Guidelines for Europe, 2nd Edition” 2000).
Methanol use and safety
Methanol can be used as a liquid feedstock for fermentation of methylotrophic organisms. While methanol is an effective carbon source, it has a low flash point, making it highly prone to ignition, and is toxic. To mitigate these risks, GMP protocols should include safety measures such as flame-proof storage and handling as well as proper ventilation to prevent the accumulation of methanol vapors (< 200ppm) (Methanol Institute 2017). Regulatory compliance is also important, especially in the food industry, where methanol use demands rigorous safety documentation and testing of final products to ensure that methanol levels are within safe limits (< 5 mg/dL) (Zamani et al. 2019).
Lignocellulosic feedstock use and safety
Lignocellulosic feedstocks, derived from agricultural residues, wood, and non-edible crops, are growing in popularity as sustainable feedstocks due to their abundance and decreased reliance on food-based substrates. However, these materials pose unique challenges, particularly during storage and hydrolysis. When stored in aerobic conditions, lignocellulosic biomass can become a breeding ground for various fungi, leading to mycotoxin contamination. Mycotoxins such as aflatoxins and ochratoxins, produced by fungi like Aspergillus and Penicillium (El-Sayed et al. 2022), can affect feedstock quality and pose serious food safety risks if they enter the fermentation process. Additionally, fungal infestation can reduce the sugar content of cellulosic feedstocks, ultimately reducing efficiency and utilization downstream (Balan 2014). Proper drying and low-moisture storage can help minimize fungal growth. Large piles of cellulosic biomass are also susceptible to spontaneous ignition, so precautionary measures such as temperature monitoring, proper ventilation, and avoiding compact storage are needed (Gray, Griffiths, and Hasko 1984).
Thermo-acidic treatments are commonly used to make the lignocellulosic sugar bioavailable for microbial fermentation. However, this process can result in the formation of furans, such as furfural, hydroxymethylfurfural (HMF), and tetrahydrofuran (THF), which are microbial growth inhibitors that can reduce productivity, increase production costs, and present safety risks for final products. Furan tolerance through genetic engineering, adaptive laboratory evolution (ALE), or using naturally tolerant microbes (e.g. Clostridium spp.) have been studied to mitigate the effects of furans, but challenges remain in the large-scale use of lignocellulosic sugars as microbial feedstocks (Jilani and Olson 2023).
